Fungal Wound Infection Risk Assessment Tool
Fill in the form to see your risk assessment and recommendations.
Fungal infections can significantly delay wound healing by forming biofilms that resist immune responses and antifungal treatments.
Common risk factors include:
- Moist wound environment
- Compromised immunity
- Recent antibiotic use
- Underlying conditions like diabetes
Early detection and targeted treatment are crucial for preventing chronic wound complications.
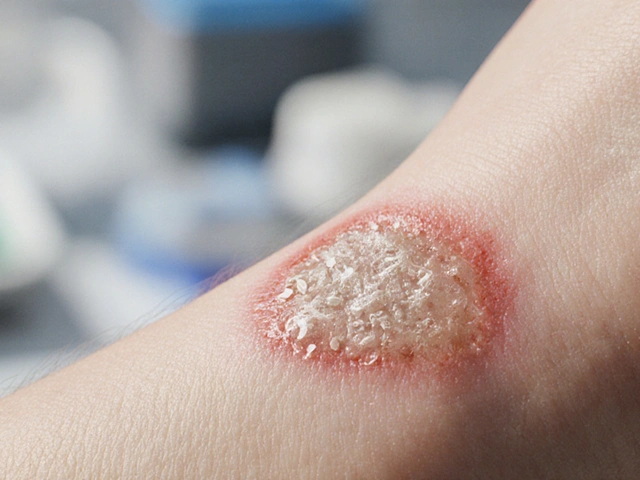
When a cut or scrape gets infected, we usually think of bacteria. But fungal infections are sneakier foes that can slow healing and turn a simple abrasion into a chronic problem. Understanding how these microscopic invaders work, which species show up most often, and what you can do to keep them out is key to faster recovery and fewer complications.
Key Takeaways
- Fungi can colonize wounds as quickly as 24‑48hours, especially in moist or immunocompromised environments.
- Common wound fungi include Candida albicans a yeast that thrives on skin and mucous membranes and Aspergillus fumigatus a mold that spreads through airborne spores.
- Fungal biofilms protect the organisms from both the immune system and topical antifungals, making chronic wounds harder to treat.
- Early diagnosis with culture or PCR and targeted antifungal therapy can restore normal healing timelines.
- Prevention hinges on wound hygiene, moisture control, and, for high‑risk patients, prophylactic measures.
Why Fungi Target Wounds
Wound tissue provides a warm, nutrient‑rich niche that many fungi love. Once the skin barrier is broken, spores or yeast cells that linger on the surface can settle into the wound bed. In a healthy adult, the immune system usually clears these invaders quickly. However, several factors tilt the balance in favor of the fungus:
- Moisture: Dressings that stay damp for too long create a perfect breeding ground.
- Compromised immunity: Diabetes, steroids, or HIV reduce the body’s ability to fight off fungal growth.
- Broad‑spectrum antibiotics: Killing off bacteria removes competition, allowing fungi to expand.
- Foreign material: Sutures, grafts, or even latex gloves can act as scaffolding for fungal colonies.
These conditions also promote the formation of biofilm a structured community of microorganisms embedded in a protective matrix, which shields fungi from immune attacks and antifungal drugs.
How Fungal Presence Alters Healing
Normal wound healing follows a predictable sequence: hemostasis, inflammation, proliferation, and remodeling. Fungal colonization disrupts several of these stages:
- Prolonged inflammation: The immune system releases cytokines trying to eradicate the fungus, extending the inflammatory phase and causing excess tissue damage.
- Impaired granulation: Fungal metabolites can inhibit fibroblast activity, slowing the formation of new connective tissue.
- Delayed epithelialization: The protective epidermal layer forms later because keratinocyte migration is hampered by inflammatory debris.
- Scar formation: Chronic inflammation often leads to excess collagen deposition, increasing the risk of hypertrophic scars.
The net result is a wound that stalls in the proliferative phase, lingering for weeks or months as a chronic wound a wound that fails to progress through the normal healing stages within 30 days.
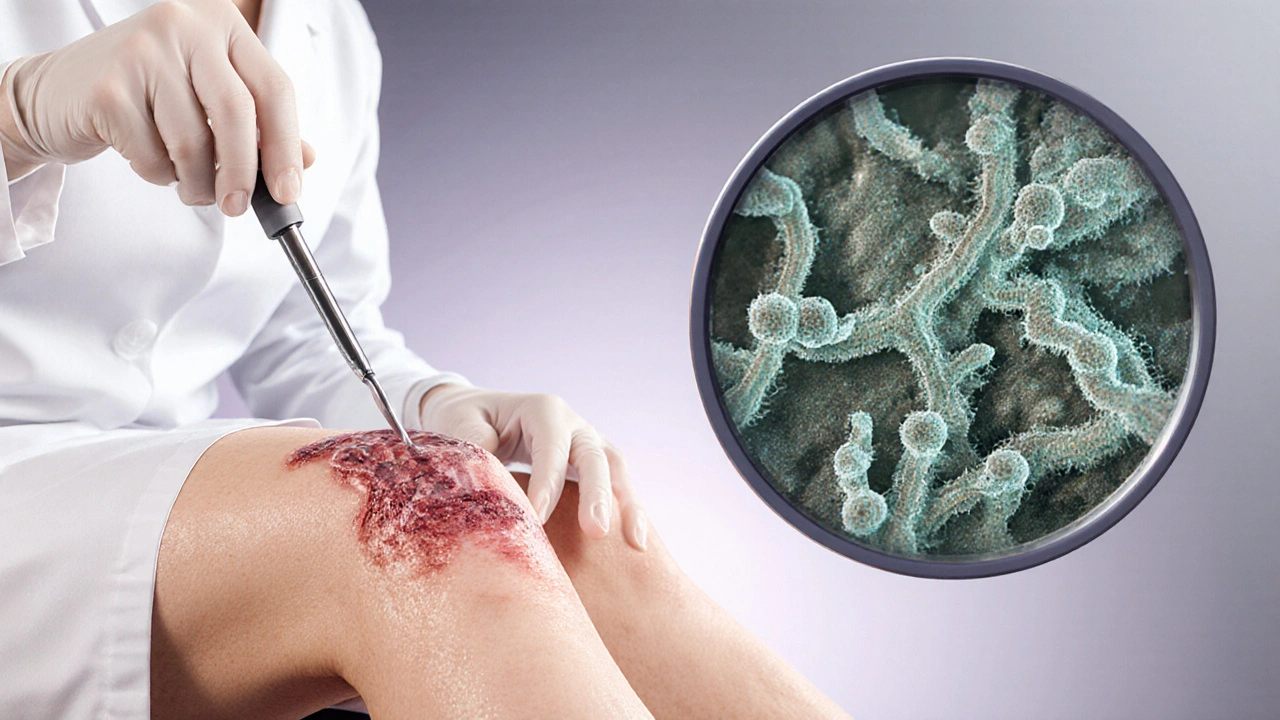
Common Fungal Culprits in Wound Cases
Not every fungus is a threat, but a handful repeatedly shows up in clinical wound cultures. Below is a brief snapshot of the most frequent players and what makes them dangerous.
- Candida albicans yeast that forms pseudohyphae, allowing tissue invasion - Often linked to diabetic foot ulcers and postoperative wounds.
- Aspergillus fumigatus mold that releases airborne conidia, thriving in humid environments - Seen in burns and trauma patients with prolonged ICU stays.
- Cryptococcus neoformans encapsulated yeast, rare but reported in immunocompromised wound infections.
- Fusarium spp. soil‑borne molds that can cause painful, necrotic lesions.
Diagnosing Fungal Wound Infections
Early detection is the difference between a quick fix and a months‑long ordeal. Here’s a practical workflow clinicians often follow:
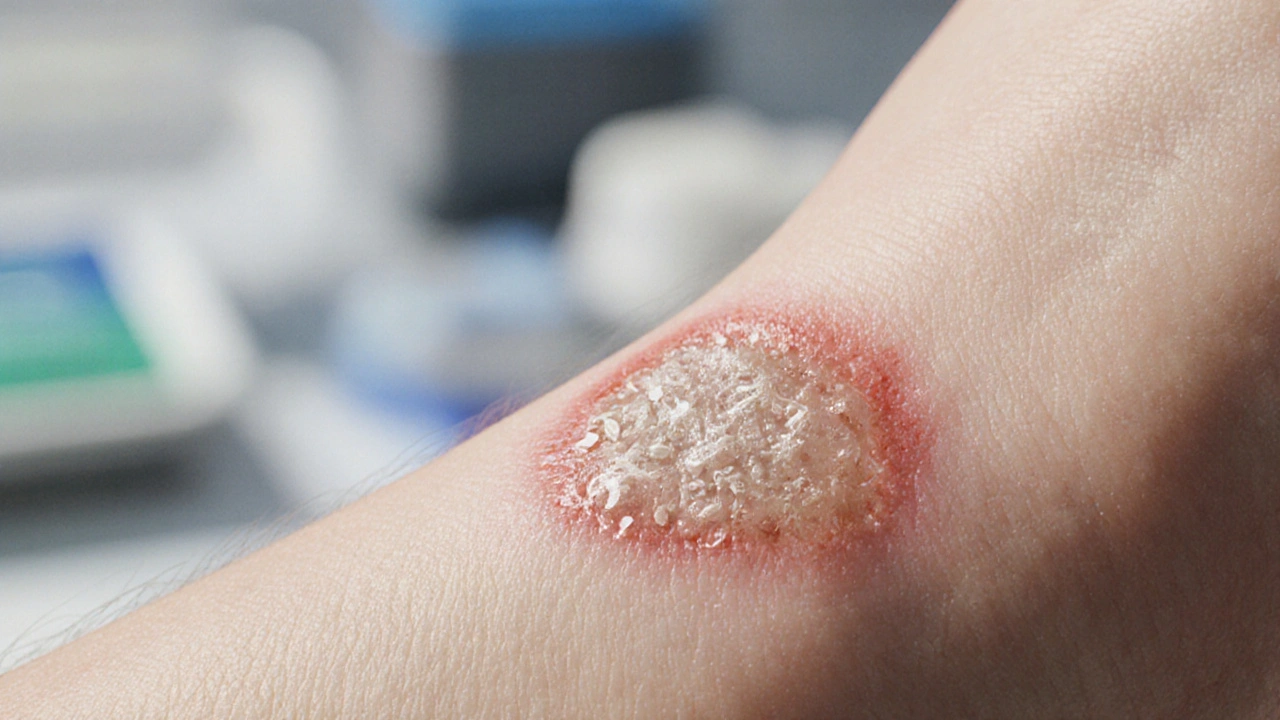
- Clinical suspicion: Look for foul odor, excessive granulation tissue, or a “wet” appearance despite standard care.
- Sample collection: Use a sterile curette or punch biopsy. Swabs alone can miss deeper fungal elements.
- Laboratory testing:
- Culture on Sabouraud dextrose agar - grows most yeasts and molds within 48-72hours.
- Histopathology with PAS or GMS stains - highlights fungal walls directly in tissue.
- PCR‑based assays - provide rapid species identification, useful for resistant strains.
- Interpretation: Combine lab results with clinical picture. A positive culture without inflammation might simply indicate colonization, not infection.
Prevention: Stopping Fungi Before They Settle
Preventing fungal colonization is easier than eradicating an established biofilm. Below are evidence‑backed steps anyone caring for a wound can implement.
- Moisture management: Use breathable, semi‑occlusive dressings that wick away excess fluid. Change dressings at least every 48hours.
- Antimicrobial stewardship: Reserve broad‑spectrum antibiotics for confirmed bacterial infections. Overuse creates an opening for fungi.
- Skin integrity: Keep surrounding skin clean, dry, and moisturized with barrier creams to stop fungal spread.
- Prophylactic antifungals: In high‑risk patients (e.g., severe burns, neutropenia), consider topical nystatin or oral fluconazole as a short‑term measure.
- Environmental control: Maintain hospital humidity below 60% and ensure proper ventilation in wound‑care units.
Treatment Strategies for Established Infections
When fungi have already taken hold, a multimodal approach works best.
- Debridement: Surgical removal of necrotic tissue reduces fungal load and disrupts biofilm.
- Targeted antifungal therapy:
- Terbinafine an allylamine effective against dermatophytes and Candida species - oral 250mg daily for 2‑4 weeks.
- Voriconazole a triazole with broad activity against Aspergillus and rare molds - loading dose 6mg/kg IV, then 4mg/kg BID.
- Topical options: nystatin cream for Candida‑predominant wounds; amphotericin B lipid complex for resistant molds.
- Adjunctive measures:
- Negative‑pressure wound therapy (NPWT) can improve perfusion and pull away biofilm matrix.
- Hyperbaric oxygen (HBO) boosts neutrophil function and may hasten fungal clearance.
Therapy length varies. For superficial Candida, 2 weeks often suffice. Invasive molds may require 6‑12 weeks plus close imaging follow‑up.
Comparing Fungal vs. Bacterial Wound Infections
| Aspect | Fungal Infection | Bacterial Infection |
|---|---|---|
| Typical onset | 24‑48h after injury, especially in moist wounds | Within hours, often rapid pus formation |
| Common organisms | Candida albicans, Aspergillus spp. | Staphylococcus aureus, Streptococcus pyogenes, Pseudomonas aeruginosa |
| Clinical clues | Excessive granulation, foul odor, non‑purulent exudate | Redness, warmth, purulent drainage |
| Response to antibiotics | Typically poor; may worsen due to disrupted flora | Usually good, unless resistant strain |
| Preferred treatment | Antifungal agents + debridement | Targeted antibiotics + wound care |
Practical Checklist for Clinicians and Caregivers
- Assess wound moisture every 24h; switch to dry‑promoting dressings if needed.
- Ask about recent antibiotic courses - high‑risk if >7days.
- Inspect for signs: yellowish exudate, persistent granulation, or foul smell.
- Collect tissue samples before starting any antifungal; avoid surface swabs alone.
- Initiate debridement promptly once fungal growth is confirmed.
- Choose antifungal based on species susceptibility - terbinafine for Candida, voriconazole for molds.
- Re‑evaluate wound size and depth weekly; extend therapy if healing plateaus.
- Educate patients on keeping the wound clean, dry, and covered - simple hygiene cuts infection risk dramatically.
Future Directions - What’s on the Horizon?
Research is buzzing around three promising areas:
- Rapid point‑of‑care PCR: Handheld devices could identify fungal DNA in under 30minutes, slashing diagnosis time.
- Anti‑biofilm peptides: Lab studies show short‑chain peptides can disrupt fungal matrices, making drugs more effective.
- Smart dressings: Materials embedded with pH sensors and controlled-release antifungals adjust therapy automatically.
These advances aim to turn fungal wound infections from a hidden nightmare into a manageable, even preventable, condition.
Frequently Asked Questions
Can a bacterial infection turn into a fungal one?
Yes. Broad‑spectrum antibiotics can wipe out normal skin flora, removing competition and allowing fungi that were merely colonizing to overgrow and cause infection.
What is the fastest way to tell if a wound has a fungal infection?
Look for a non‑purulent, often watery exudate with a distinct smell, plus excessive granulation tissue that doesn’t improve with standard antibacterial care. Confirm with a tissue culture or PCR.
Are over‑the‑counter antifungal creams enough for wound infections?
Topical creams may help early, superficial Candida infections, but deeper or mold‑related wounds usually need oral agents and surgical debridement.
How long should antifungal treatment last for a chronic wound?
Treatment typically continues for at least 2‑4 weeks after the wound shows positive signs of healing, but refractory cases can require 6‑12 weeks plus close monitoring.
Can lifestyle changes reduce the risk of fungal wound infections?
Absolutely. Maintaining good blood sugar control, avoiding prolonged moisture, and limiting unnecessary antibiotic use are proven ways to keep fungi at bay.





October 5, 2025 AT 17:59
Nicole Koshen
Thanks for the thorough overview! I appreciate how you broke down the risk factors, especially the role of moisture and recent antibiotic use. It’s vital for clinicians to assess wound environment daily and adjust dressings accordingly. The inclusion of a simple risk calculator is a nice practical touch that can be used at the bedside. Overall, great job presenting complex information in an accessible format.
October 8, 2025 AT 18:12
Ed Norton
Very useful info
November 1, 2025 AT 13:26
Karen Misakyan
One must acknowledge that fungal pathogens, unlike their bacterial counterparts, possess a unique affinity for keratinized substrates, thereby complicating the wound milieu. The literature consistently underscores the propensity of Candida albicans to form pseudohyphal structures that facilitate tissue invasion. Moreover, Aspergillus fumigatus spores, being aerosolized, can readily colonize moist dressings if sterility is compromised. The biofilm matrix, composed of extracellular polysaccharides, not only impedes immune cell penetration but also diminishes antifungal susceptibility. Consequently, clinicians should prioritize rapid diagnostic modalities, such as PCR, to circumvent delays inherent to culture. It is also prudent to integrate serial histopathological evaluations when clinical suspicion persists despite negative cultures. In immunocompromised hosts, prophylactic antifungal regimens are justified, although stewardship principles must guide their judicious use. Lastly, interdisciplinary collaboration between infectious disease specialists and wound care teams optimizes therapeutic outcomes. The systematic approach delineated herein aligns with current evidence-based guidelines and merits widespread adoption.
December 1, 2025 AT 15:39
Amy Robbins
Oh great, another post telling us to keep wounds dry while we’re all over‑watering our lawns. Sure, “smart dressings” will magically solve it, but let’s not forget that the government loves a good bio‑film story to push more pharma. Also, who decided antibiotics are the villain? If you ask me, it’s the media.
December 31, 2025 AT 17:52
Michelle Zhao
While the article extols the virtues of moisture control, one might argue that a modestly moist environment is essential for epithelial migration. The blanket condemnation of “damp” dressings ignores the nuanced physiology of granulation tissue. Furthermore, the insinuation that antifungal prophylaxis is merely a “short‑term measure” understates the risk in patients with chronic immunosuppression. It is also worth noting that the cited risk matrix oversimplifies the interplay of systemic comorbidities. In light of these considerations, a more individualized algorithm would be preferable.
January 30, 2026 AT 20:06
Eric Parsons
Fungal wound infections are often the underappreciated culprits behind delayed healing, and understanding their biology is the first step toward effective management.
First, fungi thrive in moist, nutrient‑rich environments, so regular assessment of wound exudate is paramount.
Second, the immunocompromised host-whether due to diabetes, steroids, or HIV-provides a fertile ground for opportunistic species like Candida and Aspergillus.
Third, the indiscriminate use of broad‑spectrum antibiotics can eradicate bacterial flora that normally competes with fungi, inadvertently encouraging fungal overgrowth.
Fourth, biofilm formation is a hallmark of chronic fungal colonization; the extracellular matrix shields organisms from both host defenses and topical agents.
Fifth, early detection hinges on recognizing atypical signs such as non‑purulent, foul‑smelling drainage and excessive granulation tissue that fails to contract.
Sixth, tissue sampling-using a sterile curette or punch biopsy-is far more reliable than swabs, which often miss deeper hyphae.
Seventh, laboratory confirmation can be expedited with PCR assays, delivering species‑level identification within hours rather than days.
Eighth, treatment must be multimodal: surgical debridement to reduce fungal load, followed by targeted systemic antifungals (e.g., fluconazole for Candida, voriconazole for molds).
Ninth, adjunctive therapies such as negative‑pressure wound therapy can improve perfusion and disrupt biofilm architecture.
Tenth, hyperbaric oxygen may enhance neutrophil function and synergize with antifungal agents.
Eleventh, duration of therapy should be guided by clinical response-generally at least two weeks beyond observable healing, but longer for deep or mold infections.
Twelfth, prevention remains the cornerstone: maintain a dry wound bed with breathable dressings, limit unnecessary antibiotics, and control systemic risk factors like hyperglycemia.
Thirteenth, for high‑risk patients, short‑term prophylaxis with topical nystatin or oral fluconazole can be considered, but stewardship principles must still apply.
Fourteenth, environmental controls in healthcare settings-humidity below 60 % and proper ventilation-reduce airborne spore burden.
Finally, continued research into rapid point‑of‑care diagnostics, anti‑biofilm peptides, and smart dressings promises to shift fungal wound infections from a dreaded complication to a manageable condition.
By integrating these strategies, clinicians can markedly improve healing trajectories and reduce the burden of chronic wounds.